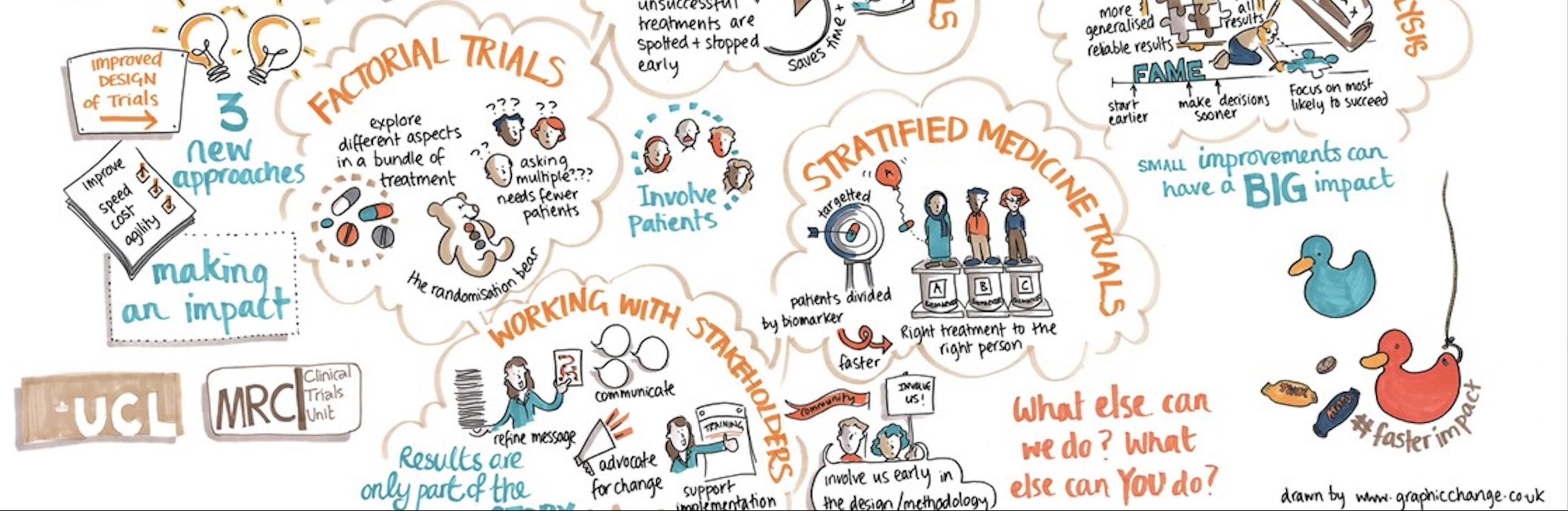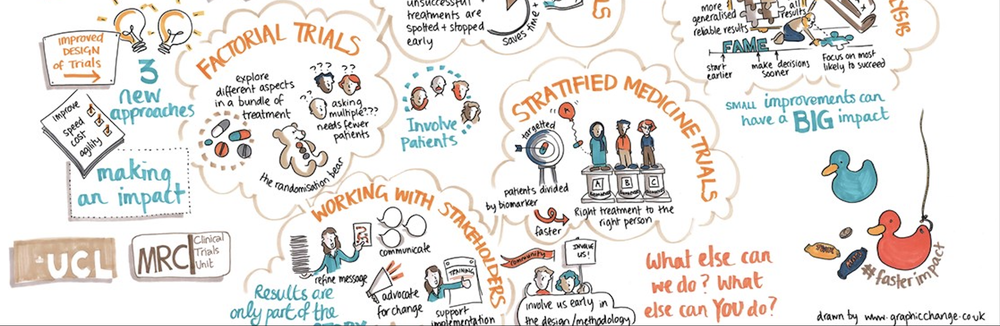
This Hub aims to provide resources on the design, conduct, analysis, and knowledge transfer and exchange for randomised controlled trials, observational studies, and meta-analyses. We will also advertise new training opportunities and short courses.
The resources shared on the Hub have been created by the MRC CTU at UCL and partners, some are for particular trials and studies and others are more generic. They may be useful for those conducting clinical trials and studies in different countries, and could be used as a template to adapt for other contexts. Country-specific regulations, and the needs, resources, expertise and local knowledge in the setting, will be important to take into account. We will do our best to ensure documents on this Hub are kept up to date. If you use any slides or training materials, please acknowledge the authors.
For further information on the MRC Clinical Trials Unit (MRC CTU) at UCL, please see our website.