Upcoming Online Short Courses at the Institute of Clinical Trials and Methodology. A few free places and discounts are offered to applicants from LMICs.
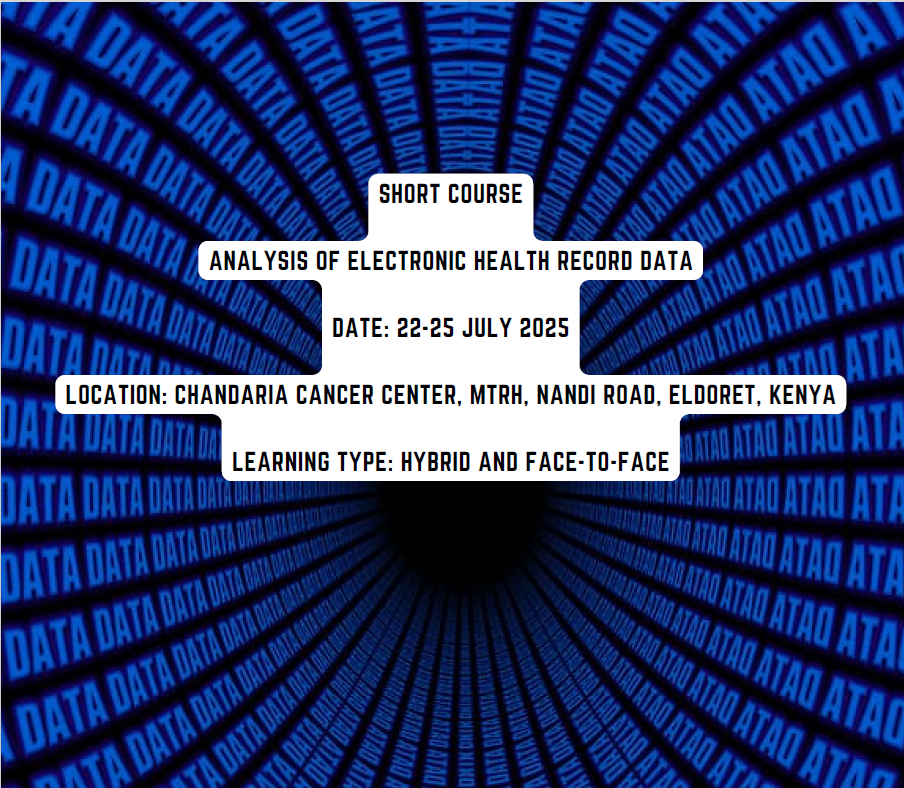
Analysis of Electronic Health Record Data Short Course
22-25 July | Hybrid and face-to-face | Chandaria Cancer Center, Eldoret, Kenya | Apply
Sub-Saharan Africa Consortium for Advanced Biostatistics training (SSACAB) in collaboration with MOI University, London School of Hygiene & Tropical Medicine Electronic Health Records Research Group & Wits School of Public Health
Who should apply?
All practicals will be conducted in R Students should have a working knowledge of how to use R prior to the course to fully engage withthe materials. There is no practical introducing that will be provided to students on how to use R. Students should be familiar withregression models, such as linear and logistic regression, and interpretation of their results.

Analysis of Electronic Health Record Data short course information sheet
APPLY FOR THE ANALYSIS OF ELECTRONIC HEALTH RECORD DATA SHORT COURSE
HIV Modelling Consortium
There are many free online courses on understanding modelling analyses and results and learning to code a model from scratch.
Find out more
The Union - Sign up for a new free online course on how to develop a research grant proposal
The Union is launching a brand-new course to empower researchers in low or middle income countries (LMICs).
Find out more
MRC Clinical Trials Unit at UCL MAMS Clinic

This is an advisory service on how to optimise the design, implementation, and analysis of your multi-arm multi-stage (MAMS) randomised platform trial. We provide advice on how to maximise the efficient of testing several treatments for you and the community.
For more information please click here
Adaptive Clinical Trial Designs

Adaptive Clinical Trial Designs A six-part online course introducing the future of clinical trials Dates 31st January – 30th November 2024 Infectious diseases are an urgent global concern, yet traditional randomised controlled trials lag behind what is needed in terms ...READ MORE
Online course for interpretation of CXRs in children with presumptive TB
Available free-of-charge to all audiences

The course is based on the Union’s Diagnostic CXR Atlas for Tuberculosis in Children: a Guide to chest X-ray Interpretation (2nd edition, 2022) that provides pragmatic guidance to healthcare professionals working in child tuberculosis. We aim to narrow the detection-treatment ...READ MORE
Online MSc Clinical Trials Courses
The UCL Institute of Clinical Trials & Methodology (ICTM) online programmes for healthcare graduates.

Join one of these exciting online programmes for healthcare graduates (pharmacy, life sciences, medicine, nursing).READ MORE
The Research Learning Lectures
Hosted by the NIHR Associate Principal Investigator (PI) Scheme

The Research Learning Lectures are a series of lectures for anyone in Health and Social Care interested in learning more about research hosted by the NIHR Associate Principal Investigator (PI) Scheme.READ MORE